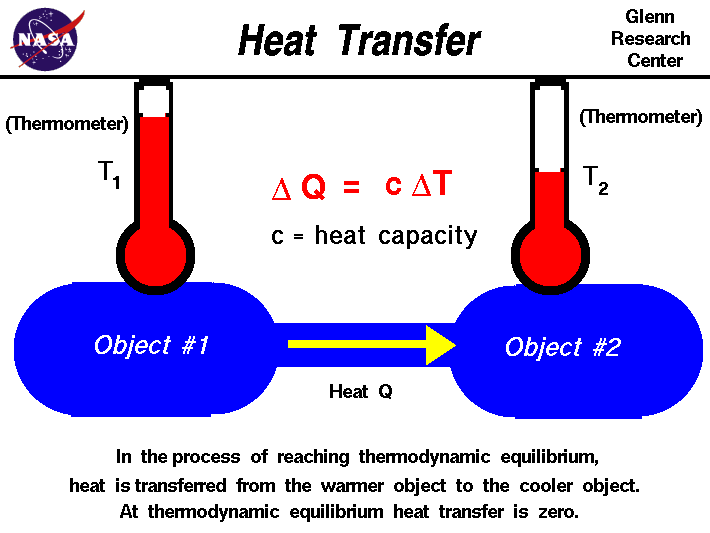
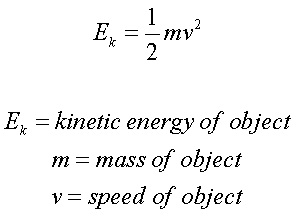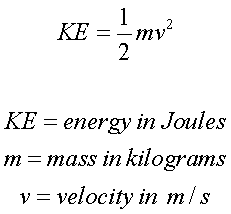The First Law of Thermodynamics
- energy change = energy at final state - energy at initial state
- E(in) - E(out) = [Q(in) - Q(out)] + [W(in) - W(out)] + [E(mass,in) - E(mass,out)] = energy change
- Energy can exist in numerous forms such as thermal, mechanical, kinetic, potential, electric, magnetic, chemical, and nuclear, and their sum constitutes the total energy, E of a system Thermodynamics deals only with the change of the total energy.
EFFICIENCY
Frequently used terms in thermodynamics to indicate how well an energy conversion or transfer process is accomplished.
OVERALL EFFICIENCY
Generator:
A device that converts mechanical energy to electrical energy.
Generator efficiency:
The ratio of the electrical power output to the mechanical power input.
Thermal efficiency of a power plant:
The ratio of the net electrical power output to the rate of fuel energy input.
ENERGY AND ENVIRONMENT
-Energy conversions affects the environment in many ways, and thus the study of energy is not complete without considering its impact on the environment.
-Pollutants emitted during the combustion of fossil fuels are responsible for smog, acid rain, and global warming.
1. Ozone and Smog
2. Acid Rain
3.The Greenhouse Effect: Global Warming and Climate Change.
3.The Greenhouse Effect: Global Warming and Climate Change.
Solutions:
1) Energy Efficient technology.
2) Renewable energy.